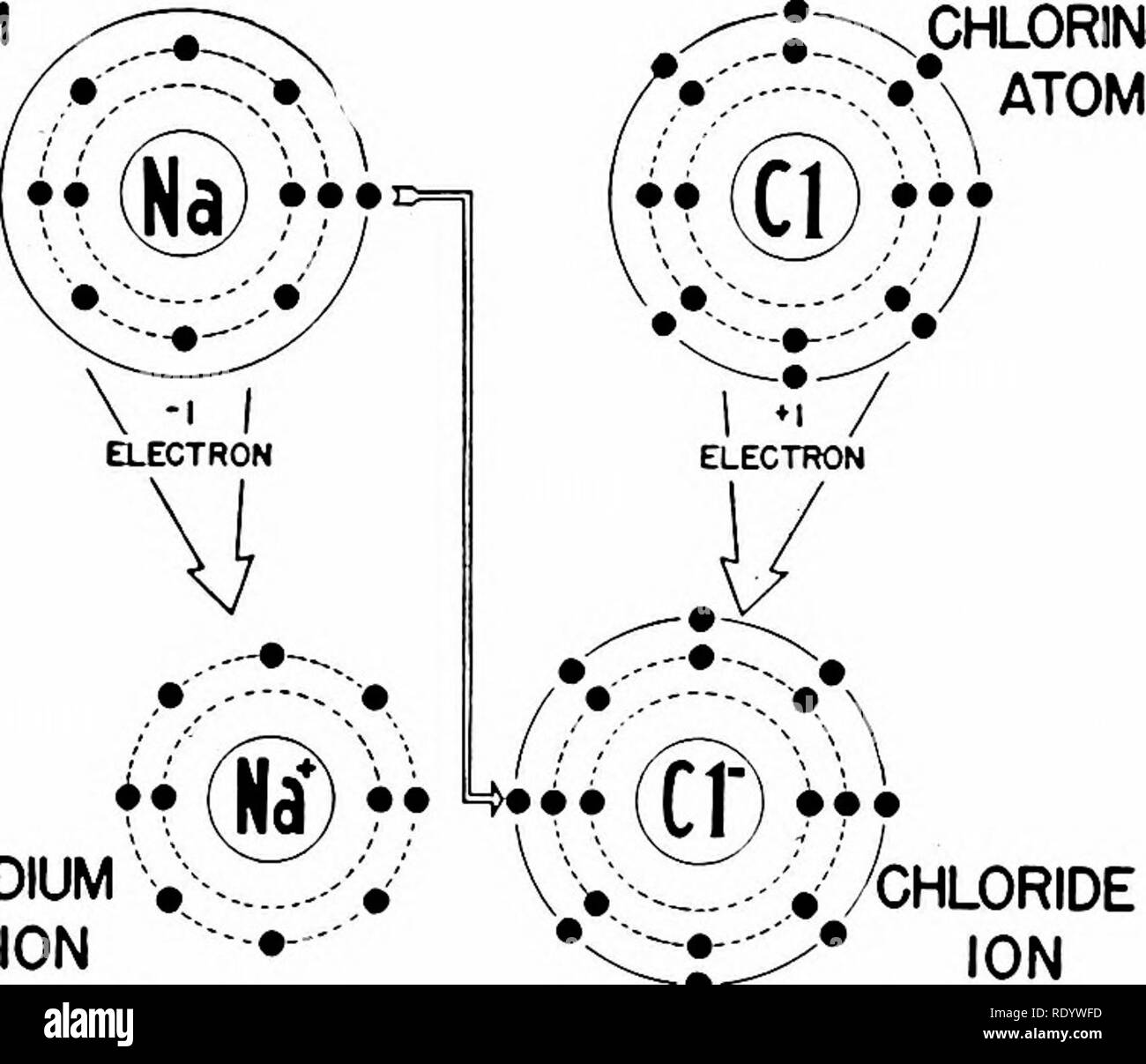
Thus, each proton and neutron has a mass of about 1 amu. Isotope: Atoms of the same element with the same atomic number, but different number of neutrons. Isotope of an element is defined by the sum of the number of protons and neutrons in its nucleus. Elements have more than one isotope with varying numbers of neutrons. Since the atomic number of calcium is 20 and the charge is positive, this means the ion has 20 - 2 or 18 electrons. Chemical Elements To be an element, a substance has to at least have protons, since these particles define the type of element.

Also found in: Thesaurus.
Related to atomic number 42: Forty two
| Noun | 1. | atomic number 42 - a polyvalent metallic element that resembles chromium and tungsten in its properties; used to strengthen and harden steel molybdenum, Mo metal, metallic element - any of several chemical elements that are usually shiny solids that conduct heat or electricity and can be formed into sheets etc. molybdenite - a mineral resembling graphite that is valued as the chief source of molybdenum and its compounds |
Based on WordNet 3.0, Farlex clipart collection. © 2003-2012 Princeton University, Farlex Inc.
 Want to thank TFD for its existence? Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content.
Want to thank TFD for its existence? Tell a friend about us, add a link to this page, or visit the webmaster's page for free fun content. Link to this page:
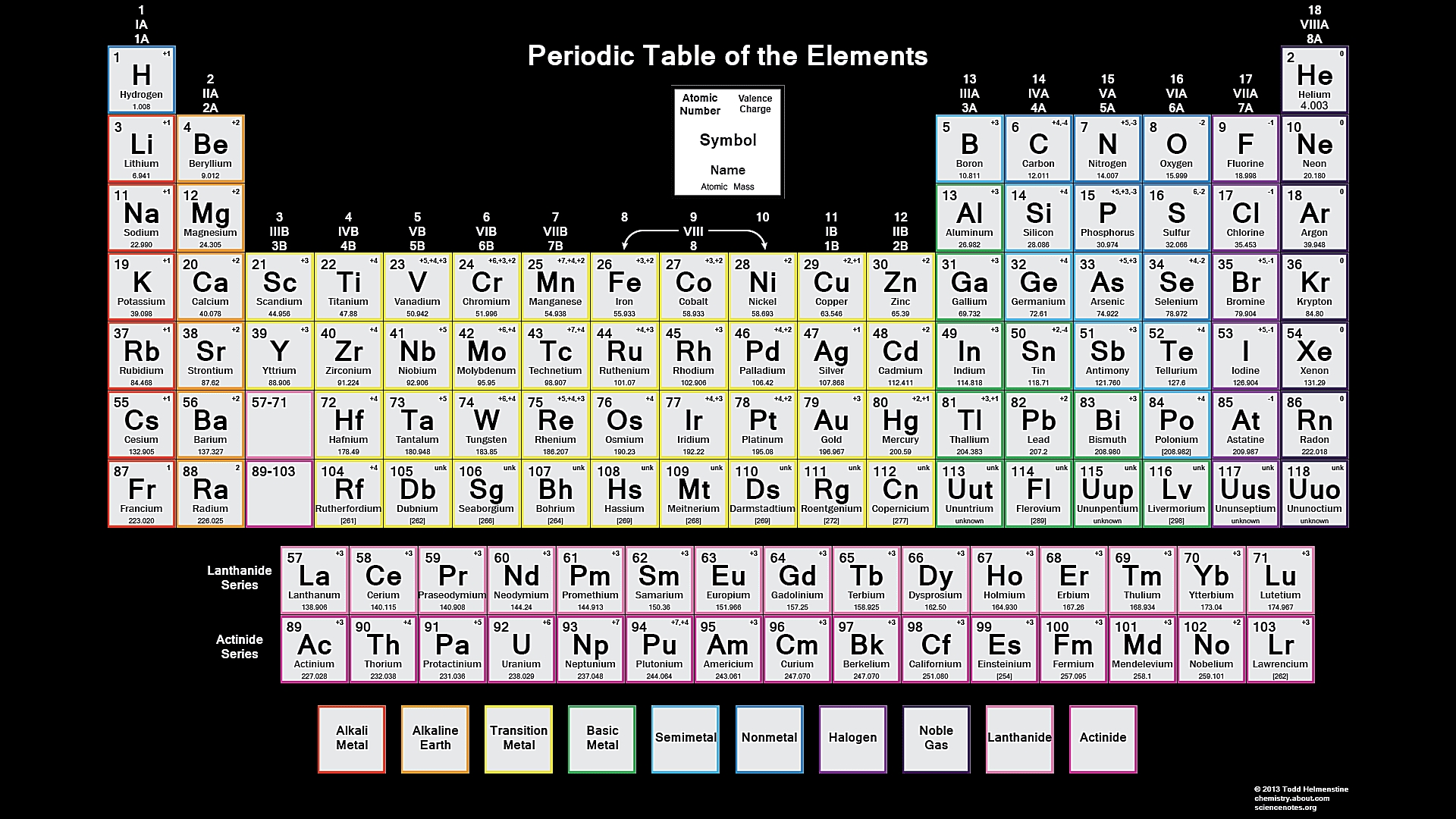
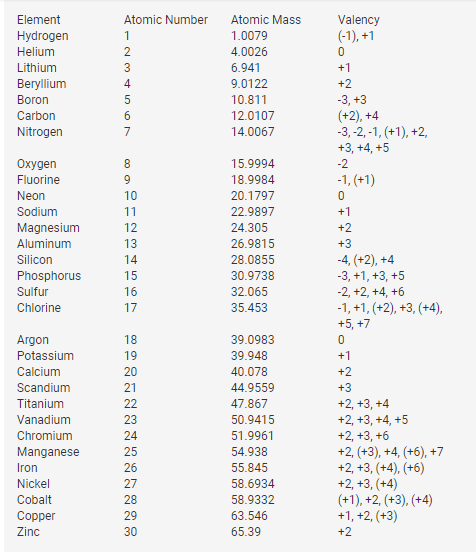
First 10 Periodic Table
